

When
Ernest Rutherford proposed the nuclear model of an atom?
a) 1803
b)
1904
c) 1911
d) 1913
Who discovered/proposed the planetary model of an atom?
a) John Dalton
b) J. J. Thomson
c) Ernest Rutherford
d) Niel Bohr
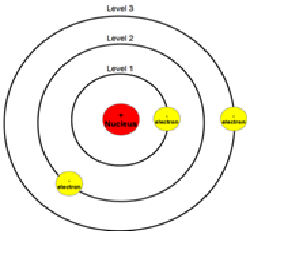
Explanation:According to the Bohr model, often referred to as a planetary model, the electrons encircle the nucleus of the atom in specific allowable paths called orbits. When the electron is in one of these orbits, its energy is fixed.
When
Niel Bohr proposed the planetary model of an atom?
a) 1803
b)
1904
c)
1911
d) 1913
Who discovered/proposed the Quantum model of an atom?
a) Erwin Schrodinger
b) J. J. Thomson
c) Ernest Rutherford
d) John Dalton
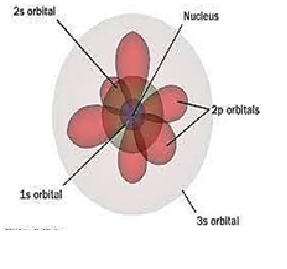
Explanation:Quantum mechanics models describe the possibility of placing electrons within an atom by describing the principal energy level, energy level, orbital (arbitrary level), and spin of. The quantum mechanics model is based on quantum theory, which states that matter has wave-like properties.
When Erwin
Schrodinger proposed the Quantum model of an atom?
a) 1803
b) 1904
c) 1911
d) 1926
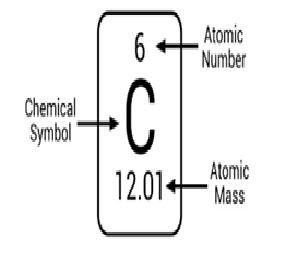
Atomic Number:
The atomic number or nuclear charge number of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number or the number of protons found in the nucleus for every atom of that element. It is represented by Z.
The number of
proton is called…….
a) atomic number
b) mass number
c) atomic mass
d) isotope number