

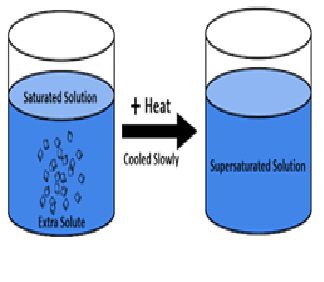
The solution in which more solute are dissolved at higher temperature than saturation point. Such solution is known as………
a) saturated solution
b) unsaturated solution
c) super saturated solution
d) all of these
In which of the following solution, the solute is unstable and forms crystals?
a) saturated solution
b) unsaturated solution
c) super saturated solution
d) all of these
Rock candy is the best example of ……….
a) saturated solution
b) unsaturated solution
c) super saturated solution
d) all of these
Explanation:A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. The recrystallization of the excess dissolved solute in a supersaturated solution can be initiated by the addition of a tiny crystal of solute, called a seed crystal.
How the
temperature generally affects the solubility of gas solutes in water?
a) gases are more soluble at higher temperature
b) gases are less soluble at higher
temperature
c) the solubility of gases does not affected by temperature
d) none of these
In which of the following water pond, more oxygen will be soluble?
a) at 5◦ C
b) at 10◦ C
c) at 20◦ C
d) at 30◦ C
In which of the following water pond, less oxygen will be soluble and fish face lack of oxygen?
a) at 0◦ C
b) at 10◦ C
c) at 20◦ C
d) at 50◦ C