

Solubility of a solute in a solvent depends on………factors mainly.
a) one
b) two
c) three
d) five
Explanation: 1. The nature of solute and solvent, 2. The temperature and
3. The pressure
How nature of solute and solvent affect the solubility?
a) polar solute dissolves in non-polar solvent
b) polar solute dissolves in polar solvent
c) non- polar solute dissolves in polar solvent
d) all of these
How nature of solute and solvent affect the solubility?
a) non-polar solute dissolves in polar solvent
b) polar solute dissolves in non-polar solvent
c) non-polar solute dissolves in non-polar solvent
d) all of these
Which of the following is general formula for the solubility of solute in a solvent?
a) solid dissolves in liquid
b) covalent solute dissolves in polar solvent
c) like dissolves like
d) all of these
Explanation:Like Dissolves Like. A simple way to predict which compounds will dissolve in other compounds is the phrase "like dissolves like". What this means is that polar compounds dissolve polar compounds, nonpolar compounds dissolve nonpolar compounds, but polar and nonpolar do not dissolve in each other.
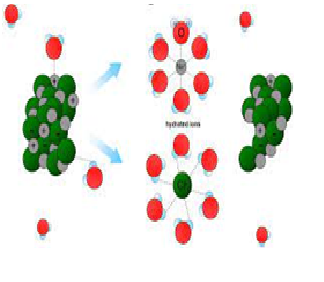
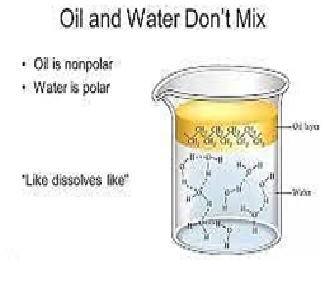
Why
oil is not miscible in water?
a) oil is polar and water is non-polar
b) oil is non-polar and water is polar
c) both oil water are non-polar
d) both oil and water are polar
Explanation:Oils and fats not have any polar part and so for them to dissolve in water they would have to break some of water s hydrogen bonds. Water will not do this so the oil is forced to stay separate from the water.
How
the temperature generally affects the solubility of solid and liquid solutes?
a) solutes are more soluble at higher
temperature
b) solutes are less soluble at higher temperature
c) the solubility of solutes does not affected by temperature
d) none of these