36.0 g

The solution in which the maximum amount solute has been dissolved in a given solvent…
a) Saturated
b) unsaturated
c) super saturated
d) none of these
Explanation:A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent. The additional solute will not dissolve in a saturated solution.
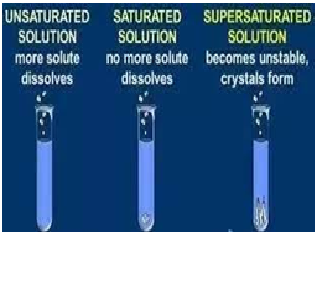
What is the
solubility of NaCl in 100 ml water?
a) 30g
b) 34g
c) 40g
d) 50g
The solubility of NaCl in 100 ml water is 34g. If 30g of NaCl is dissolved in 100ml water, what will be the solution?
a) Saturated
b) unsaturated
c) super saturated
d) none of these
The solubility of NaCl in 100 ml water is 34g. If 34g of NaCl is dissolved in 100ml water, what will be the solution?
a) Saturated
b) unsaturated
c) super saturated
d) none
The solubility of NaCl in 100 ml water is 34g. If 40g of NaCl is dissolved in 100ml water, what will be happen?
a) all salt will be dissolve
b) 4 gram salt will remain insoluble
c) no salt will be dissolved
d) none of these
Factors Affecting The Solubility Of A Solute In A Solvent:
Factors affecting solubility ·Temperature Polarity · Pressure Molecular size Stirring increases the speed of dissolving.
36 g
by at 2025-03-13 23:12:02